Are you over 18 and want to see adult content?
More Annotations

A complete backup of justiceadmin.org
Are you over 18 and want to see adult content?

A complete backup of alimentude.com.br
Are you over 18 and want to see adult content?

A complete backup of unitechelectronics.com
Are you over 18 and want to see adult content?
A complete backup of museumfoundation.org
Are you over 18 and want to see adult content?
A complete backup of truegrublord.tumblr.com
Are you over 18 and want to see adult content?
A complete backup of e-gitarre-lernen.blogspot.com
Are you over 18 and want to see adult content?
Favourite Annotations
A complete backup of visitdandenongranges.com.au
Are you over 18 and want to see adult content?
A complete backup of diclofenacovercounter.com
Are you over 18 and want to see adult content?
A complete backup of supportdriverprinter.com
Are you over 18 and want to see adult content?

A complete backup of lafeteducourt.com
Are you over 18 and want to see adult content?

A complete backup of pasarpoker1.club
Are you over 18 and want to see adult content?
Text
JOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non CHEMOCENTRYX TO HOST VIRTUAL R&D DAY ON APRIL 14, 2021 MOUNTAIN VIEW, Calif., April 07, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced that it will host a virtual R&D Day on Wednesday, April 14, 2021 beginning at 12:30 p.m. ET.The event will feature a panel of key opinion leaders, a testimonial from a patient living with ANCA-associated vasculitis and members of the ChemoCentryx Management team. CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CCX507 – CHEMOCENTRYX CCX507 Also in the area of inflammatory bowel disease, our drug candidate CCX507 is a second-generation inhibitor of the chemokine receptor known as CCR9. CCX507 is selective for CCR9 relative to all other chemokine receptors, orally bioavailable, and has an excellent preclinical safety profile. We have completed Phase I clinical development and we plan TERMS AND CONDITIONS OF USE The following terms and conditions concern your use of any web site owned or operated by ChemoCentryx, Inc. (“the ChemoCentryx sites”). By accessing, using or downloading any information and/or materials from a web site owned or operated by ChemoCentryx, Inc., you agree to follow and be bound by these terms and conditions (the“Terms”).
CHEMOCENTRYX IDENTIFIES NOVEL ORALLY ADMINISTERED IMMUNE 835 Industrial Ave. Suite 600 San Carlos, CA 94070. Phone: +1.650.210.2900. Fax: +1.650.210.2910. Email: info@chemocentryx.com CHEMOCENTRYX AND VFMCRP PROVIDE TOPLINE RESULTS FROM Vifor Pharma Group is a global pharmaceuticals company. It aims to become the global leader in iron deficiency, nephrology and cardio-renal therapies. The company is a partner of choice for pharmaceuticals and innovative patient-focused solutions. SEC FILING | CHEMOCENTRYX, INC. You may also obtain a separate proxy statement or annual report without charge by sending a written request to ChemoCentryx, Inc., 850 Maude Avenue, Mountain View, CA 94043, Attention: Corporate Secretary, or by calling us at 650-210-2900. CHEMOCENTRYX PRESENTS DATA ON CCX168, AN ORALLY 850 Maude Avenue Mountain View, CA 94043. Phone: +1.650.210.2900. Fax: +1.650.210.2910. Email: info@chemocentryx.com CHEMOCENTRYXSTOCK INFORMATIONNEWS RELEASESPARTNERINGOTHER INFLAMMATORY AND AUTOIMMUNE DISEASES People Deserve Better Medicine. At ChemoCentryx, we believe our patients deserve more than the standard of care available today. So, we are developing our broad portfolio of new medicines using a novel approach to better provide patients with safe, convenient and more effective therapies. OVERVIEW | CHEMOCENTRYX, INC.HISTORIC STOCK LOOKUPINVESTOR FAQSFINANCIALS & FILINGSTH17 DRIVEN DISEASES AND CCR6 Overview. ChemoCentryx is a biopharmaceutical company focused exclusively on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. Our drug candidates target specific chemokines or chemoattractant receptors, thereby blocking the inflammatory response driven OVERVIEW – CHEMOCENTRYX Therapeutic Area Drug/ Target Indication Preclinical Phase 1 CHEMOCENTRYX ANNOUNCES RESULTS OF FDA ADVISORY COMMITTEE SAN CARLOS, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced the outcome of the U.S. Food and Drug Administration (“FDA”) Arthritis Advisory Committee (“Committee”) on avacopan for the treatment of Antineutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis (or AAV). In the final part of the public meeting, the Committee voted onJOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CHEMOCENTRYX REPORTS FOURTH QUARTER AND FULL YEAR 2020 -- The New England Journal of Medicine highlights results of ADVOCATE Phase III trial of avacopan in ANCA-associated vasculitis ---- Applications for regulatory approval of avacopan in ANCA-associated vasculitis under review in the U.S. (PDUFA goal date of July 7, 2021), the E.U. (decision expected in H2 2021) and Japan---- Topline data from AURORA Phase II clinical trial of avacopan in CHEMOCENTRYX TO HOST VIRTUAL R&D DAY ON APRIL 14, 2021 MOUNTAIN VIEW, Calif., April 07, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced that it will host a virtual R&D Day on Wednesday, April 14, 2021 beginning at 12:30 p.m. ET.The event will feature a panel of key opinion leaders, a testimonial from a patient living with ANCA-associated vasculitis and members of the ChemoCentryx Management team. CHEMOCENTRYX SUBMITS NEW DRUG APPLICATION TO THE U.S. FDA MOUNTAIN VIEW, Calif., July 09, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (NASDAQ: CCXI) today confirmed that the Company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for avacopan for the treatment of patients with ANCA-associated vasculitis. The Company’s NDA submission is supported by the results of its pivotal Phase CHEMOCENTRYX IDENTIFIES NOVEL SMALL MOLECULE C5AR ChemoCentryx Identifies Novel Small Molecule C5aR Antagonist Mountain View, CA - May 5, 2009 - ChemoCentryx, Inc., today announced that it has identified an orally bioavailable small molecule antagonist of the C5a receptor, CCX168, as a candidate for clinical development. The acceptance of the molecule as a development candidate triggered a $5.0 million milestone payment from the alliance CHEMOCENTRYXSTOCK INFORMATIONNEWS RELEASESPARTNERINGOTHER INFLAMMATORY AND AUTOIMMUNE DISEASES People Deserve Better Medicine. At ChemoCentryx, we believe our patients deserve more than the standard of care available today. So, we are developing our broad portfolio of new medicines using a novel approach to better provide patients with safe, convenient and more effective therapies. OVERVIEW | CHEMOCENTRYX, INC.HISTORIC STOCK LOOKUPINVESTOR FAQSFINANCIALS & FILINGSTH17 DRIVEN DISEASES AND CCR6 Overview. ChemoCentryx is a biopharmaceutical company focused exclusively on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. Our drug candidates target specific chemokines or chemoattractant receptors, thereby blocking the inflammatory response driven OVERVIEW – CHEMOCENTRYX Therapeutic Area Drug/ Target Indication Preclinical Phase 1 CHEMOCENTRYX ANNOUNCES RESULTS OF FDA ADVISORY COMMITTEE SAN CARLOS, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced the outcome of the U.S. Food and Drug Administration (“FDA”) Arthritis Advisory Committee (“Committee”) on avacopan for the treatment of Antineutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis (or AAV). In the final part of the public meeting, the Committee voted onJOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CHEMOCENTRYX REPORTS FOURTH QUARTER AND FULL YEAR 2020 -- The New England Journal of Medicine highlights results of ADVOCATE Phase III trial of avacopan in ANCA-associated vasculitis ---- Applications for regulatory approval of avacopan in ANCA-associated vasculitis under review in the U.S. (PDUFA goal date of July 7, 2021), the E.U. (decision expected in H2 2021) and Japan---- Topline data from AURORA Phase II clinical trial of avacopan in CHEMOCENTRYX TO HOST VIRTUAL R&D DAY ON APRIL 14, 2021 MOUNTAIN VIEW, Calif., April 07, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced that it will host a virtual R&D Day on Wednesday, April 14, 2021 beginning at 12:30 p.m. ET.The event will feature a panel of key opinion leaders, a testimonial from a patient living with ANCA-associated vasculitis and members of the ChemoCentryx Management team. CHEMOCENTRYX SUBMITS NEW DRUG APPLICATION TO THE U.S. FDA MOUNTAIN VIEW, Calif., July 09, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (NASDAQ: CCXI) today confirmed that the Company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for avacopan for the treatment of patients with ANCA-associated vasculitis. The Company’s NDA submission is supported by the results of its pivotal Phase CHEMOCENTRYX IDENTIFIES NOVEL SMALL MOLECULE C5AR ChemoCentryx Identifies Novel Small Molecule C5aR Antagonist Mountain View, CA - May 5, 2009 - ChemoCentryx, Inc., today announced that it has identified an orally bioavailable small molecule antagonist of the C5a receptor, CCX168, as a candidate for clinical development. The acceptance of the molecule as a development candidate triggered a $5.0 million milestone payment from the allianceMANAGEMENT TEAM
Management Team Dr. Schall is the founder of our company and has served as our President, Chief Executive Officer and Director since we commenced operations in 1997 and was appointed Chairman of the Board in April 2012. From 1993 to 1997, Dr. Schall worked at the DNAX Research Institute, a division of Schering-Plough Corporation.NEWS RELEASES
News Releases. MOUNTAIN VIEW, Calif. , April 22, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. , (Nasdaq: CCXI), today announced that the Company's first quarter 2021 financial results will be released after market close on Thursday, April 29, 2021 . ChemoCentryx executive management will host a conference call and. CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler CHEMOCENTRYX AND VFMCRP ANNOUNCE TOPLINE DATA FROM PHASE MOUNTAIN VIEW, Calif. and ST GALLEN, CH, May 18, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (NASDAQ: CCXI) and Vifor Fresenius Medical Care Renal Pharma (VFMCRP) today announced topline data from a forty-six (46) patient Phase II dose-ranging trial in the orphan kidney disorder, primary Focal Segmental Glomerulosclerosis (FSGS). The LUMINA-1 trial tested CCX140, an CHEMOCENTRYX ANNOUNCES FDA ACCEPTANCE OF THE AVACOPAN NEW -- FDA sets PDUFA goal date of July 7, 2021 --MOUNTAIN VIEW, Calif., Sept. 17, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced that the U.S. Food and Drug Administration (FDA) has accepted the Company’s New Drug Application (NDA) for avacopan, an orally-administered selective complement 5a receptor inhibitor, for the treatment of ANCA-Associated TERMS AND CONDITIONS OF USE The following terms and conditions concern your use of any web site owned or operated by ChemoCentryx, Inc. (“the ChemoCentryx sites”). By accessing, using or downloading any information and/or materials from a web site owned or operated by ChemoCentryx, Inc., you agree to follow and be bound by these terms and conditions (the“Terms”).
CHEMOCENTRYX PHASE III ADVOCATE TRIAL OF AVACOPAN IN ANCA 835 Industrial Ave. Suite 600 San Carlos, CA 94070. Phone: +1.650.210.2900. Fax: +1.650.210.2910. Email: info@chemocentryx.com CHEMOCENTRYX AND VFMCRP PROVIDE TOPLINE RESULTS FROM Vifor Pharma Group is a global pharmaceuticals company. It aims to become the global leader in iron deficiency, nephrology and cardio-renal therapies. The company is a partner of choice for pharmaceuticals and innovative patient-focused solutions. CHEMOCENTRYX PRESENTS DATA ON CCX168, AN ORALLY 850 Maude Avenue Mountain View, CA 94043. Phone: +1.650.210.2900. Fax: +1.650.210.2910. Email: info@chemocentryx.com CHEMOCENTRYX IDENTIFIES NOVEL SMALL MOLECULE C5AR ChemoCentryx Identifies Novel Small Molecule C5aR Antagonist Mountain View, CA - May 5, 2009 - ChemoCentryx, Inc., today announced that it has identified an orally bioavailable small molecule antagonist of the C5a receptor, CCX168, as a candidate for clinical development. The acceptance of the molecule as a development candidate triggered a $5.0 million milestone payment from the alliance CHEMOCENTRYXSTOCK INFORMATIONNEWS RELEASESPARTNERINGOTHER INFLAMMATORY AND AUTOIMMUNE DISEASES People Deserve Better Medicine. At ChemoCentryx, we believe our patients deserve more than the standard of care available today. So, we are developing our broad portfolio of new medicines using a novel approach to better provide patients with safe, convenient and more effective therapies. OVERVIEW | CHEMOCENTRYX, INC.HISTORIC STOCK LOOKUPINVESTOR FAQSFINANCIALS & FILINGSTH17 DRIVEN DISEASES AND CCR6 Overview. ChemoCentryx is a biopharmaceutical company focused exclusively on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. Our drug candidates target specific chemokines or chemoattractant receptors, thereby blocking the inflammatory response driven OVERVIEW – CHEMOCENTRYX Therapeutic Area Drug/ Target Indication Preclinical Phase 1JOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non CHEMOCENTRYX ANNOUNCES RESULTS OF FDA ADVISORY COMMITTEE SAN CARLOS, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced the outcome of the U.S. Food and Drug Administration (“FDA”) Arthritis Advisory Committee (“Committee”) on avacopan for the treatment of Antineutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis (or AAV). In the final part of the public meeting, the Committee voted on ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CHEMOCENTRYX REPORTS FOURTH QUARTER AND FULL YEAR 2020 -- The New England Journal of Medicine highlights results of ADVOCATE Phase III trial of avacopan in ANCA-associated vasculitis ---- Applications for regulatory approval of avacopan in ANCA-associated vasculitis under review in the U.S. (PDUFA goal date of July 7, 2021), the E.U. (decision expected in H2 2021) and Japan---- Topline data from AURORA Phase II clinical trial of avacopan in CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler SEC FILING | CHEMOCENTRYX, INC. If the filing person has previously filed a statement on Schedule 13G to report the acquisition which is the subject of this Schedule 13D, and is filing this schedule because of §§ 240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box.o VIFOR PHARMA LICENSES RIGHTS TO COMMERCIALIZE CHEMOCENTRYX - Vifor Pharma to market drug in selected territories outside the US, ChemoCentryx responsible for worldwide development of CCX168 - - ChemoCentryx to receive USD 85 million comprising USD 60 million in cash and USD 25 million equity investment; Deal also includes regulatory and commercial milestone payments and royalties on net sales to ChemoCentryx in the Vifor Pharma CHEMOCENTRYXSTOCK INFORMATIONNEWS RELEASESPARTNERINGOTHER INFLAMMATORY AND AUTOIMMUNE DISEASES People Deserve Better Medicine. At ChemoCentryx, we believe our patients deserve more than the standard of care available today. So, we are developing our broad portfolio of new medicines using a novel approach to better provide patients with safe, convenient and more effective therapies. OVERVIEW | CHEMOCENTRYX, INC.HISTORIC STOCK LOOKUPINVESTOR FAQSFINANCIALS & FILINGSTH17 DRIVEN DISEASES AND CCR6 Overview. ChemoCentryx is a biopharmaceutical company focused exclusively on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. Our drug candidates target specific chemokines or chemoattractant receptors, thereby blocking the inflammatory response driven OVERVIEW – CHEMOCENTRYX Therapeutic Area Drug/ Target Indication Preclinical Phase 1JOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non CHEMOCENTRYX ANNOUNCES RESULTS OF FDA ADVISORY COMMITTEE SAN CARLOS, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced the outcome of the U.S. Food and Drug Administration (“FDA”) Arthritis Advisory Committee (“Committee”) on avacopan for the treatment of Antineutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis (or AAV). In the final part of the public meeting, the Committee voted on ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CHEMOCENTRYX REPORTS FOURTH QUARTER AND FULL YEAR 2020 -- The New England Journal of Medicine highlights results of ADVOCATE Phase III trial of avacopan in ANCA-associated vasculitis ---- Applications for regulatory approval of avacopan in ANCA-associated vasculitis under review in the U.S. (PDUFA goal date of July 7, 2021), the E.U. (decision expected in H2 2021) and Japan---- Topline data from AURORA Phase II clinical trial of avacopan in CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler SEC FILING | CHEMOCENTRYX, INC. If the filing person has previously filed a statement on Schedule 13G to report the acquisition which is the subject of this Schedule 13D, and is filing this schedule because of §§ 240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box.o VIFOR PHARMA LICENSES RIGHTS TO COMMERCIALIZE CHEMOCENTRYX - Vifor Pharma to market drug in selected territories outside the US, ChemoCentryx responsible for worldwide development of CCX168 - - ChemoCentryx to receive USD 85 million comprising USD 60 million in cash and USD 25 million equity investment; Deal also includes regulatory and commercial milestone payments and royalties on net sales to ChemoCentryx in the Vifor Pharma OVERVIEW – CHEMOCENTRYX Overview ChemoCentryx is a biopharmaceutical company focused on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer, primarily focused on orphan and rare diseases. We balance our passion for science with the deep-seated belief that patients suffering from serious diseases deserve more than incremental CONTACT US – CHEMOCENTRYX Contact Us ChemoCentryx Headquarters 835 Industrial Ave. Suite 600 San Carlos, CA 94070 Main: +1.650.210.2900MANAGEMENT TEAM
Management Team Dr. Schall is the founder of our company and has served as our President, Chief Executive Officer and Director since we commenced operations in 1997 and was appointed Chairman of the Board in April 2012. From 1993 to 1997, Dr. Schall worked at the DNAX Research Institute, a division of Schering-Plough Corporation. ORPHAN AND RARE DISEASES Orphan and Rare Diseases ANCA Associated Vasculitis (AAV) ANCA Associated Vasculitis (AAV) is a rare, severe, and often fatal autoimmune disease that is caused by autoantibodies called anti-neutrophil cytoplasmic antibodies and is characterized by inflammation that can affect many different organ systems, and commonly involves the kidneys. AAV affects approximately 40,000 peoplein the US
CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler CHEMOCENTRYX ANNOUNCES FDA ACCEPTANCE OF THE AVACOPAN NEW -- FDA sets PDUFA goal date of July 7, 2021 --MOUNTAIN VIEW, Calif., Sept. 17, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced that the U.S. Food and Drug Administration (FDA) has accepted the Company’s New Drug Application (NDA) for avacopan, an orally-administered selective complement 5a receptor inhibitor, for the treatment of ANCA-Associated TERMS AND CONDITIONS OF USE The following terms and conditions concern your use of any web site owned or operated by ChemoCentryx, Inc. (“the ChemoCentryx sites”). By accessing, using or downloading any information and/or materials from a web site owned or operated by ChemoCentryx, Inc., you agree to follow and be bound by these terms and conditions (the“Terms”).
CHEMOCENTRYX SUBMITS NEW DRUG APPLICATION TO THE U.S. FDA MOUNTAIN VIEW, Calif., July 09, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (NASDAQ: CCXI) today confirmed that the Company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for avacopan for the treatment of patients with ANCA-associated vasculitis. The Company’s NDA submission is supported by the results of its pivotal Phase CHEMOCENTRYX AND VFMCRP ANNOUNCE POSITIVE TOPLINE DATA The Investor Relations website contains information about ChemoCentryx, Inc. 's business for stockholders, potential investors, and financial analysts. CHEMOCENTRYX AND VFMCRP PROVIDE TOPLINE RESULTS FROM Vifor Pharma Group is a global pharmaceuticals company. It aims to become the global leader in iron deficiency, nephrology and cardio-renal therapies. The company is a partner of choice for pharmaceuticals and innovative patient-focused solutions. CHEMOCENTRYXSTOCK INFORMATIONNEWS RELEASESPARTNERINGOTHER INFLAMMATORY AND AUTOIMMUNE DISEASES People Deserve Better Medicine. At ChemoCentryx, we believe our patients deserve more than the standard of care available today. So, we are developing our broad portfolio of new medicines using a novel approach to better provide patients with safe, convenient and more effective therapies. OVERVIEW | CHEMOCENTRYX, INC.HISTORIC STOCK LOOKUPINVESTOR FAQSFINANCIALS & FILINGSTH17 DRIVEN DISEASES AND CCR6 Overview. ChemoCentryx is a biopharmaceutical company focused exclusively on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. Our drug candidates target specific chemokines or chemoattractant receptors, thereby blocking the inflammatory response driven OVERVIEW – CHEMOCENTRYX Therapeutic Area Drug/ Target Indication Preclinical Phase 1JOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non CHEMOCENTRYX ANNOUNCES RESULTS OF FDA ADVISORY COMMITTEE SAN CARLOS, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced the outcome of the U.S. Food and Drug Administration (“FDA”) Arthritis Advisory Committee (“Committee”) on avacopan for the treatment of Antineutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis (or AAV). In the final part of the public meeting, the Committee voted on ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CHEMOCENTRYX REPORTS FOURTH QUARTER AND FULL YEAR 2020 -- The New England Journal of Medicine highlights results of ADVOCATE Phase III trial of avacopan in ANCA-associated vasculitis ---- Applications for regulatory approval of avacopan in ANCA-associated vasculitis under review in the U.S. (PDUFA goal date of July 7, 2021), the E.U. (decision expected in H2 2021) and Japan---- Topline data from AURORA Phase II clinical trial of avacopan in CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler SEC FILING | CHEMOCENTRYX, INC. If the filing person has previously filed a statement on Schedule 13G to report the acquisition which is the subject of this Schedule 13D, and is filing this schedule because of §§ 240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box.o VIFOR PHARMA LICENSES RIGHTS TO COMMERCIALIZE CHEMOCENTRYX - Vifor Pharma to market drug in selected territories outside the US, ChemoCentryx responsible for worldwide development of CCX168 - - ChemoCentryx to receive USD 85 million comprising USD 60 million in cash and USD 25 million equity investment; Deal also includes regulatory and commercial milestone payments and royalties on net sales to ChemoCentryx in the Vifor Pharma CHEMOCENTRYXSTOCK INFORMATIONNEWS RELEASESPARTNERINGOTHER INFLAMMATORY AND AUTOIMMUNE DISEASES People Deserve Better Medicine. At ChemoCentryx, we believe our patients deserve more than the standard of care available today. So, we are developing our broad portfolio of new medicines using a novel approach to better provide patients with safe, convenient and more effective therapies. OVERVIEW | CHEMOCENTRYX, INC.HISTORIC STOCK LOOKUPINVESTOR FAQSFINANCIALS & FILINGSTH17 DRIVEN DISEASES AND CCR6 Overview. ChemoCentryx is a biopharmaceutical company focused exclusively on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. Our drug candidates target specific chemokines or chemoattractant receptors, thereby blocking the inflammatory response driven OVERVIEW – CHEMOCENTRYX Therapeutic Area Drug/ Target Indication Preclinical Phase 1JOB POSTINGS
As an equal opportunity employer, ChemoCentryx, Inc. is committed to a diverse workforce. Employment decisions regarding recruitment and selection will be made without discrimination based on race, color, religion, national origin, gender, age, sexual orientation, physical or mental disability, genetic information or characteristic, gender identity and expression, veteran status, or other non CHEMOCENTRYX ANNOUNCES RESULTS OF FDA ADVISORY COMMITTEE SAN CARLOS, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced the outcome of the U.S. Food and Drug Administration (“FDA”) Arthritis Advisory Committee (“Committee”) on avacopan for the treatment of Antineutrophil Cytoplasmic Autoantibody (ANCA)-associated vasculitis (or AAV). In the final part of the public meeting, the Committee voted on ORPHAN AND RARE DISEASES Orphan and Rare Diseases While orphan and rare diseases affect thousands of people, many of these people are not well served by today’s standards of care. Oftentimes approaches to their conditions are not targeted enough, so in some cases treatments may make patients more ill than they would be without them. In other cases, short-termresults
CHEMOCENTRYX REPORTS FOURTH QUARTER AND FULL YEAR 2020 -- The New England Journal of Medicine highlights results of ADVOCATE Phase III trial of avacopan in ANCA-associated vasculitis ---- Applications for regulatory approval of avacopan in ANCA-associated vasculitis under review in the U.S. (PDUFA goal date of July 7, 2021), the E.U. (decision expected in H2 2021) and Japan---- Topline data from AURORA Phase II clinical trial of avacopan in CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler SEC FILING | CHEMOCENTRYX, INC. If the filing person has previously filed a statement on Schedule 13G to report the acquisition which is the subject of this Schedule 13D, and is filing this schedule because of §§ 240.13d-1(e), 240.13d-1(f) or 240.13d-1(g), check the following box.o VIFOR PHARMA LICENSES RIGHTS TO COMMERCIALIZE CHEMOCENTRYX - Vifor Pharma to market drug in selected territories outside the US, ChemoCentryx responsible for worldwide development of CCX168 - - ChemoCentryx to receive USD 85 million comprising USD 60 million in cash and USD 25 million equity investment; Deal also includes regulatory and commercial milestone payments and royalties on net sales to ChemoCentryx in the Vifor Pharma OVERVIEW – CHEMOCENTRYX Overview ChemoCentryx is a biopharmaceutical company focused on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer, primarily focused on orphan and rare diseases. We balance our passion for science with the deep-seated belief that patients suffering from serious diseases deserve more than incremental CONTACT US – CHEMOCENTRYX Contact Us ChemoCentryx Headquarters 835 Industrial Ave. Suite 600 San Carlos, CA 94070 Main: +1.650.210.2900MANAGEMENT TEAM
Management Team Dr. Schall is the founder of our company and has served as our President, Chief Executive Officer and Director since we commenced operations in 1997 and was appointed Chairman of the Board in April 2012. From 1993 to 1997, Dr. Schall worked at the DNAX Research Institute, a division of Schering-Plough Corporation. ORPHAN AND RARE DISEASES Orphan and Rare Diseases ANCA Associated Vasculitis (AAV) ANCA Associated Vasculitis (AAV) is a rare, severe, and often fatal autoimmune disease that is caused by autoantibodies called anti-neutrophil cytoplasmic antibodies and is characterized by inflammation that can affect many different organ systems, and commonly involves the kidneys. AAV affects approximately 40,000 peoplein the US
CHEMOCENTRYX ANNOUNCES PROPOSED PUBLIC OFFERING OF COMMON MOUNTAIN VIEW, Calif., June 10, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc. (Nasdaq:CCXI) announced today that it has commenced an underwritten public offering of its common stock. In connection with this offering, ChemoCentryx plans to grant the underwriters a 30-day option to purchase up to an additional 15 percent of the number of shares sold. . SVB Leerink and Piper Sandler CHEMOCENTRYX ANNOUNCES FDA ACCEPTANCE OF THE AVACOPAN NEW -- FDA sets PDUFA goal date of July 7, 2021 --MOUNTAIN VIEW, Calif., Sept. 17, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (Nasdaq: CCXI), today announced that the U.S. Food and Drug Administration (FDA) has accepted the Company’s New Drug Application (NDA) for avacopan, an orally-administered selective complement 5a receptor inhibitor, for the treatment of ANCA-Associated TERMS AND CONDITIONS OF USE The following terms and conditions concern your use of any web site owned or operated by ChemoCentryx, Inc. (“the ChemoCentryx sites”). By accessing, using or downloading any information and/or materials from a web site owned or operated by ChemoCentryx, Inc., you agree to follow and be bound by these terms and conditions (the“Terms”).
CHEMOCENTRYX SUBMITS NEW DRUG APPLICATION TO THE U.S. FDA MOUNTAIN VIEW, Calif., July 09, 2020 (GLOBE NEWSWIRE) -- ChemoCentryx, Inc., (NASDAQ: CCXI) today confirmed that the Company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for avacopan for the treatment of patients with ANCA-associated vasculitis. The Company’s NDA submission is supported by the results of its pivotal Phase CHEMOCENTRYX AND VFMCRP ANNOUNCE POSITIVE TOPLINE DATA The Investor Relations website contains information about ChemoCentryx, Inc. 's business for stockholders, potential investors, and financial analysts. CHEMOCENTRYX AND VFMCRP PROVIDE TOPLINE RESULTS FROM Vifor Pharma Group is a global pharmaceuticals company. It aims to become the global leader in iron deficiency, nephrology and cardio-renal therapies. The company is a partner of choice for pharmaceuticals and innovative patient-focused solutions.Skip to content
Great Science. Great Medicine.* About
* Overview
* Management Team
* Board of Directors* Partnering
* Contact Us
* Pipeline
* Overview
* Orphan and Rare Diseases * Chronic Kidney Disease * Immuno-Oncology and Other Therapeutic Areas * Preclinical Candidates * Other Inflammatory and Autoimmune Diseases* Vercirnon
* CCX507
* Th17 Driven Diseases and CCR6* Diseases
* Orphan and Rare Diseases * Chronic Kidney Disease* Immuno-Oncology
* Other Inflammatory and Autoimmune Diseases* Science
* Investors
* Overview
* News Releases
* Events & Presentations * Financials & Filings* SEC Filings
* Stock Information
* Historic Stock Lookup * Investment Calculator* Analyst Coverage
* Corporate Governance* Management Team
* Board of Directors * Committee Composition* Investor FAQs
* Contact Us
* Careers
* Culture
* Benefits
* Job Postings
* About
* Overview
* Management Team
* Board of Directors* Partnering
* Contact Us
* Pipeline
* Overview
* Orphan and Rare Diseases * Chronic Kidney Disease * Immuno-Oncology and Other Therapeutic Areas * Preclinical Candidates * Other Inflammatory and Autoimmune Diseases* Vercirnon
* CCX507
* Th17 Driven Diseases and CCR6* Diseases
* Orphan and Rare Diseases * Chronic Kidney Disease* Immuno-Oncology
* Other Inflammatory and Autoimmune Diseases* Science
* Investors
* Overview
* News Releases
* Events & Presentations * Financials & Filings* SEC Filings
* Stock Information
* Historic Stock Lookup * Investment Calculator* Analyst Coverage
* Corporate Governance* Management Team
* Board of Directors * Committee Composition* Investor FAQs
* Contact Us
* Careers
* Culture
* Benefits
* Job Postings
HomepageEddy Mejia
2021-03-05T21:21:56+00:00__
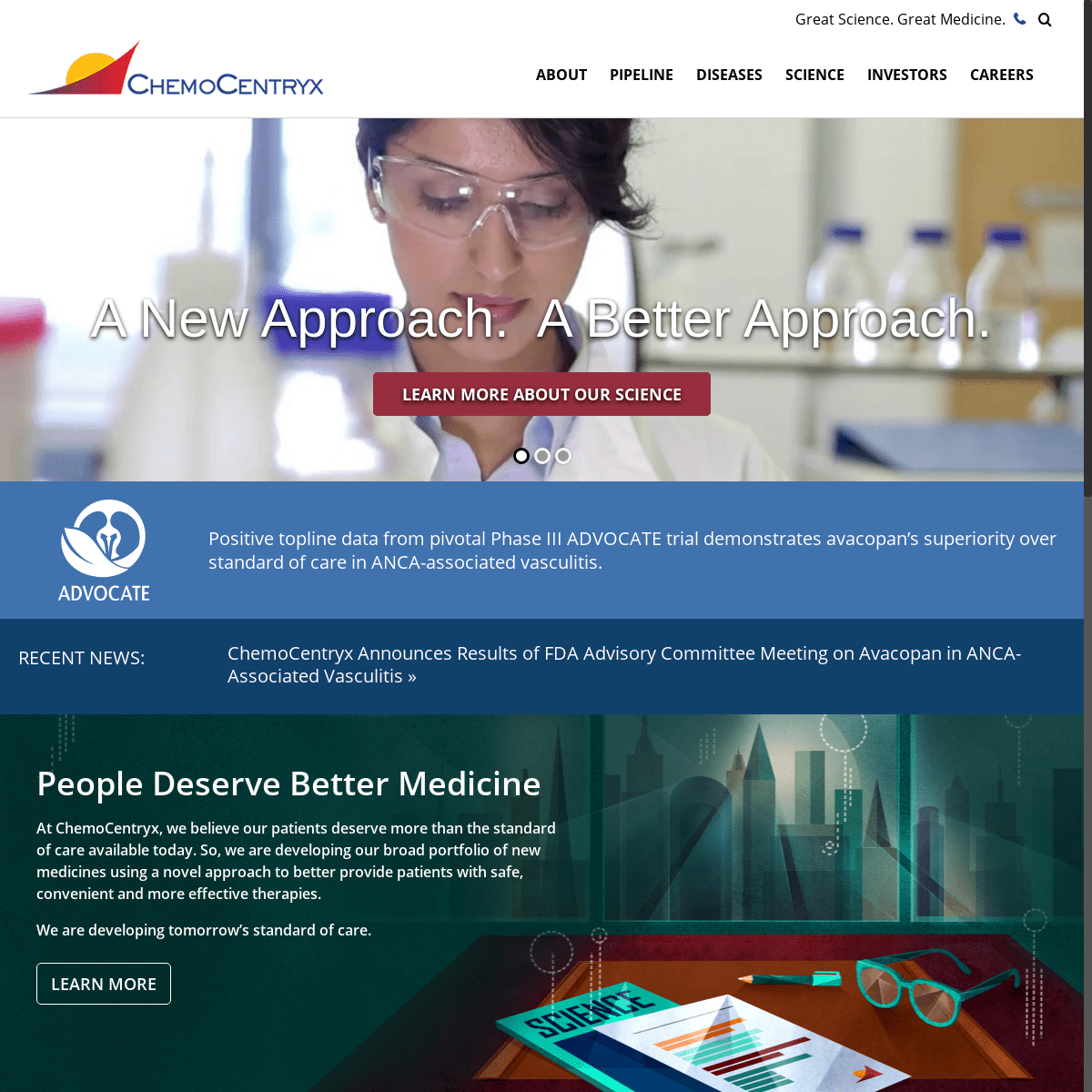
A New Approach. A Better Approach. LEARN MORE ABOUT OUR SCIENCE People Deserve Better MedicineSHARE OUR PASSION
Not Just a Longer Life, a Better Life.LEARN MORE
A New Approach. A Better Approach. LEARN MORE ABOUT OUR SCIENCE People Deserve Better MedicineSHARE OUR PASSION
Not Just a Longer Life, a Better Life.LEARN MORE
Positive topline data from pivotal Phase III ADVOCATE trial demonstrates avacopan’s superiority over standard of care in ANCA-associated vasculitis.RECENT NEWS:
ChemoCentryx Announces Results of FDA Advisory Committee Meeting on Avacopan in ANCA-Associated Vasculitis »NOW ENROLLING:
.A Phase 3 clinical trial to evaluate the safety and efficacy of avacopan in patients with ANCA-vasculitis » Clinical study evaluating CCX140 in Focal Segmental Glomerulosclerosis (FSGS) with and without Nephrotic Syndrome – Now Enrolling.RECENT NEWS:
ChemoCentryx Announces Results of FDA Advisory Committee Meeting on Avacopan in ANCA-Associated Vasculitis »ChemoCentryx
Announces Results of FDA Advisory Committee Meeting on Avacopan in ANCA-Associated Vasculitis » PEOPLE DESERVE BETTER MEDICINE AT CHEMOCENTRYX, WE BELIEVE OUR PATIENTS DESERVE MORE THAN THE STANDARD OF CARE AVAILABLE TODAY. SO, WE ARE DEVELOPING OUR BROAD PORTFOLIO OF NEW MEDICINES USING A NOVEL APPROACH TO BETTER PROVIDE PATIENTS WITH SAFE, CONVENIENT AND MORE EFFECTIVE THERAPIES. WE ARE DEVELOPING TOMORROW’S STANDARD OF CARE.Learn more
NOT JUST A LONGER LIFE, A BETTER LIFE PEOPLE SUFFERING FROM SERIOUS MEDICAL CONDITIONS WANT MORE THAN INCREMENTAL IMPROVEMENTS. LIKE ALL OF US, THEY WANT TO LIVE LONGER, MORE ENJOYABLE LIVES. THAT’S WHY WHEN OTHERS SAY, “THIS IS THE BEST WE CAN DO,” WE SAY, “WE CAN DO BETTER.” WE ARE DEVELOPING A BETTER WAY TO TREAT DISEASE.Share our passion
A NEW APPROACH. A BETTER APPROACH. OUR VISION IS SIMPLE. WE BELIEVE THAT A TARGETED BIOLOGICAL SYSTEM—THE CHEMOATTRACTANT NETWORK—IS THE KEY TO LONGER, BETTER LIVES FOR THOUSANDS OF PEOPLE SUFFERING FROM SERIOUS DISEASE. AND WE INTEND TO DELIVER THAT QUALITY OF LIFE TO PATIENTS THROUGH A PASSIONFOR GREAT SCIENCE.
OUR GREAT SCIENCE LEADS TO GREAT MEDICINE.Learn more
COMPANY
* Overview
* Management Team
* Board of Directors* Partnering
* Contact Us
PIPELINE
* Overview
* Orphan and Rare Diseases * Chronic Kidney Disease * Immuno-Oncology and Other Therapeutic Areas * Other Inflammatory and Autoimmune DiseasesDISEASES
* Orphan and Rare Diseases * Chronic Kidney Disease* Immuno-Oncology
* Other Inflammatory and Autoimmune DiseasesSCIENCE
INVESTORS
CAREERS
* Culture
* Benefits
* Job Postings
CONTACT INFORMATION
835 Industrial Ave.
Suite 600
San Carlos, CA 94070 Phone: +1.650.210.2900 Fax: +1.650.210.2910 Email: info@chemocentryx.com � 2021 ChemoCentryx, Inc. | Terms and Conditions of Use| Privacy Policy
Go to Top
Details
Copyright © 2024 ArchiveBay.com. All rights reserved. Terms of Use | Privacy Policy | DMCA | 2021 | Feedback | Advertising | RSS 2.0